Compare elements’ isotherms¶
Code: #115-000
File: apps/van_der_waals/compare_elements.ipynb
The aim of this Notebook is to compare the isotherms of different elements.
Interface¶
The main interface (main_block_115_000) is divided in two VBox: left_block and right_block. left_block consists of four bqplot Figures and right_block contains fig_115_001.
[1]:
from IPython.display import Image
Image(filename='../../static/images/apps/115-000_1.png')
[1]:
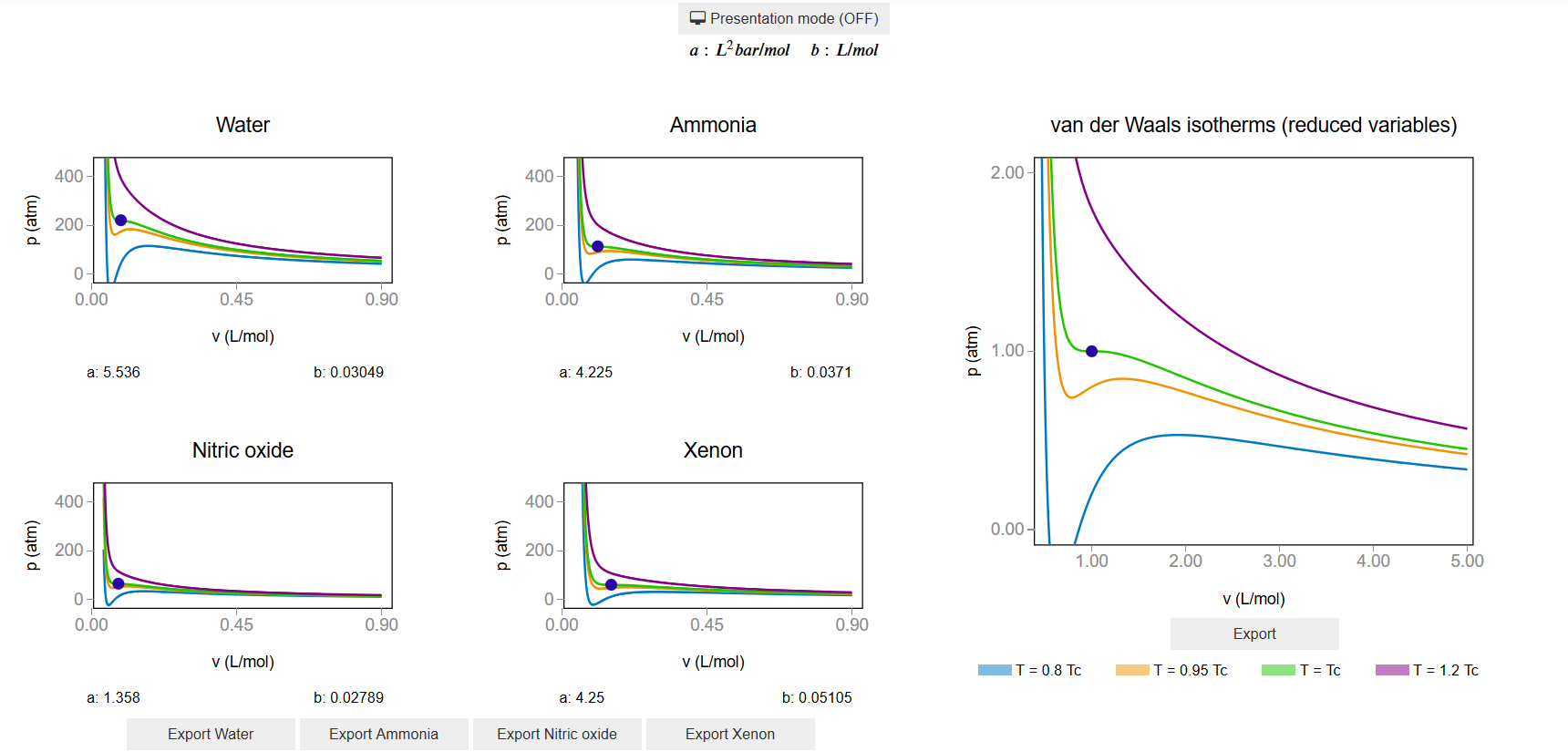
CSS¶
A custom css file is used to improve the interface of this application. It can be found here.
[2]:
from IPython.display import HTML
display(HTML("<head><link rel='stylesheet' type='text/css' href='./../../static/custom.css'></head>"))
display(HTML("<style>.container { width:100% !important; }</style>"))
Packages¶
[3]:
from bqplot import *
import bqplot as bq
import bqplot.marks as bqm
import bqplot.scales as bqs
import bqplot.axes as bqa
import ipywidgets as widgets
import urllib.parse
import webbrowser
import sys
Physical functions¶
This are the functions that have a physical meaning:
calculate_criticget_absolute_isothermsget_relative_isothermsbar_to_atm
[4]:
def calculate_critic(a, b):
"""
This function calculates the critic point
(p_c, v_c, T_c) from given a and b parameters of
the Van der Waals equation of state for real gases.
:math:`(P + a \\frac{n^2}{V^2})(V - nb) = nRT`
:math:`p_c = \\frac{a}{27 b^2}`
:math:`v_c = 3b`
:math:`T_c = \\frac{8a}{27 b R}`
Args:
a: Term related with the attraction between particles in
L^2 bar/mol^2.\n
b: Term related with the volume that is occupied by one
mole of the molecules in L/mol.\n
Returns:
p_c: Critical pressure in bar.\n
v_c: Critical volume in L/mol.\n
T_c: Critical tenperature in K.\n
"""
if b == 0.0:
return None
k_B = 1.3806488e-23 #m^2 kg s^-2 K^-1
N_A = 6.02214129e23
R = 0.082 * 1.01325 #bar L mol^-1 K^-1
p_c = a/27.0/(b**2)
v_c = 3.0*b
T_c = 8.0*a/27.0/b/R
return p_c, v_c, T_c
[5]:
def get_absolute_isotherms(a, b, v_values, T_values):
"""This function calculates the theoretical p(v, T) plane
(in absolute coordinates) according to van der Waals
equation of state from a given range of volumes
and tenperatures.
Args:
a: Term related with the attraction between particles in
L^2 bar/mol^2.\n
b: Term related with the volume that is occupied by one
mole of the molecules in L/mol.\n
v_values: An array containing the values of v
for which the isotherms must be calculated.\n
T_values: An array containing the values of T for which
the isotherms must be calculated.\n
Returns:
isotherms: A list consisted of numpy arrays containing the
pressures of each isotherm.
"""
isotherms = []
R = 0.082 * 1.01325 #bar L mol^-1 K^-1
for T in T_values:
isot = []
for v in v_values:
p = R*T/(v - b) - (a/v**2)
isot = np.append(isot, p)
isotherms.append(isot)
return isotherms
[6]:
def get_relative_isotherms(v_range, T_range):
"""This function calculates the theoretical p(v, T) plane
(in reduced coordinates) according to van der Waals
equation of state from a given range of volumes
and tenperatures.
Args:
v_range: An array containing the values of v
(in reduced coordinates)for which the isotherms must be
calculated.\n
T_range: An array containing the values of T
(in reduced coordinates)for which the isotherms must be
calculated.\n
Returns:
isotherms: A list consisted of numpy arrays containing the
pressures of each isotherm.
"""
isotherms = []
for T in T_range:
p_R = []
for v in v_range:
val = (8.0/3.0*T/(v - 1.0/3.0) - 3.0/v**2)
p_R = np.append(p_R, val)
isotherms.append(p_R)
return isotherms
[7]:
def bar_to_atm(p_values):
"""This function changes the pressures of an array
form bars to atm.
Args:
p_values: List consisted of pressures in bars.\n
Returns:
p_values: List consisted of pressures in atm.\n
"""
p_values = np.array(p_values) * 0.9869
return p_values
Main interface¶
[ ]:
#(a, b, element's name)
parameters = [(5.536, 0.03049, 'Water'),
(4.225, 0.0371, 'Ammonia'),
(1.358, 0.02789, 'Nitric oxide'),
(4.25, 0.05105, 'Xenon')]
colors = ['#0079c4','#f09205','#21c400', '#850082']
#I want to show the same range in v so you can compare the isotherms of all the elements
#so, let's calculate the critic point of the first one and use as a reference for the rest
p_c1, v_c1, T_c1 = calculate_critic(parameters[0][0], parameters[0][1])
v_values = np.linspace(0.8*parameters[0][1], 10*v_c1, 500)
scale_x = bqs.LinearScale(min = min(v_values), max = max(v_values))
scale_y = bqs.LinearScale(min = 0.0, max = 2.0*p_c1)
axis_x = bqa.Axis(
scale=scale_x,
tick_format='.2f',
tick_style={'font-size': '15px'},
tick_values=[0, 0.45, 0.9],
grid_lines = 'none',
grid_color = '#8e8e8e',
label='v (L/mol)',
label_location='middle',
label_style={'stroke': 'black', 'default-size': 35},
label_offset='50px'
)
axis_y = bqa.Axis(
scale=scale_y,
tick_format='.0f',
tick_style={'font-size': '15px'},
tick_values=[0, 200, 400],
grid_lines = 'none',
grid_color = '#8e8e8e',
orientation='vertical',
label='p (atm)',
label_location='middle',
label_style={'stroke': 'red', 'default_size': 35},
label_offset='50px'
)
main_block_115_000 = widgets.VBox(
[],
layout=widgets.Layout(width='100%')
)
left_block = widgets.VBox(
[],
layout=widgets.Layout(width='60%')
)
right_block = widgets.VBox(
[],
layout=widgets.Layout(width='40%')
)
h_block_1 = widgets.HBox([])
left_block.children = [h_block_1]
if len(parameters) > 3:
h_block_2 = widgets.HBox([])
left_block.children = [
h_block_1,
h_block_2
]
figures = []
for i in range(len(parameters)):
elem = parameters[i]
a = elem[0]
b = elem[1]
name = elem[2]
p_c, v_c, T_c = calculate_critic(a, b)
T_values = [0.8*T_c, 0.95*T_c, T_c, 1.2*T_c]
T_values_str = [str(t) for t in T_values]
v_values = np.linspace(b+0.01, 0.9, 500)
isotherms = get_absolute_isotherms(a, b, v_values, T_values)
isotherms = bar_to_atm(isotherms)
block = widgets.VBox(
[],
layout=widgets.Layout(width='100%')
)
marks = []
lines = bqm.Lines(
x = [v_values for elem in isotherms],
y = isotherms,
scales = {'x': scale_x, 'y': scale_y},
opacities = [1.0],
visible = True, #True, #t == '1.00',
colors = colors,
labels = T_values_str,
)
critical_point = bqm.Scatter(
name = '',
x = [v_c],
y = [p_c],
scales = {'x': scale_x, 'y': scale_y},
default_opacities = [1.0],
visible = True,
colors = ['#2807a3'],
)
marks = [
lines,
critical_point
]
fig = Figure(
title=name,
marks=marks,
axes=[axis_x, axis_y],
animation_duration=0,
legend_location='top-right',
background_style= {'fill': 'white', 'stroke': 'black'},
min_aspect_ratio=1.0,
fig_margin=dict(top=80, bottom=60, left=80, right=30),
toolbar = True,
layout = widgets.Layout(width='90%', height='250px')
)
figures.append(fig)
block.children = [
fig,
widgets.HBox([
widgets.HTML(value='a: '+str(a)),
widgets.HTML(value='b: '+str(b)),
],
layout=widgets.Layout(
align_self='center',
justify_content='space-around',
width='100%'
)
)
]
if i > 1:
h_block_2.children = h_block_2.children + (block,)
else:
h_block_1.children = h_block_1.children + (block,)
v_values = np.linspace(0.45, 5.0, 500)
T_values = [0.8, 0.95, 1.0, 1.2]
T_values_str = [str(t) for t in T_values]
relative_isotherms = get_relative_isotherms(v_values, T_values)
scale_x = bqs.LinearScale(min = 0.45, max = 5.0)
scale_y = bqs.LinearScale(min = 0.0, max = 2.0)
axis_x = bqa.Axis(
scale=scale_x,
tick_format='0.2f',
tick_style={'font-size': '15px'},
tick_values=[1,2,3,4,5],
grid_lines = 'none',
grid_color = '#8e8e8e',
label='v (L/mol)',
label_location='middle',
label_style={'stroke': 'black', 'default-size': 35},
label_offset='50px'
)
axis_y = bqa.Axis(
scale=scale_y,
tick_format='0.2f',
tick_style={'font-size': '15px'},
tick_values=[0,1,2],
grid_lines = 'none',
grid_color = '#8e8e8e',
orientation='vertical',
label='p (atm)',
label_location='middle',
label_style={'stroke': 'red', 'default_size': 35},
label_offset='50px'
)
fig_115_001 = Figure(
title='van der Waals isotherms (reduced variables)',
marks=[],
axes=[axis_x, axis_y],
animation_duration=0,
legend_location='top-right',
background_style= {'fill': 'white', 'stroke': 'black'},
min_aspect_ratio=1.0,
fig_margin=dict(top=80, bottom=60, left=80, right=30),
toolbar = True,
layout = widgets.Layout(width='90%')
)
lines = bqm.Lines(
x = [v_values for elem in relative_isotherms],
y = relative_isotherms,
scales = {'x': scale_x, 'y': scale_y},
opacities = [1.0],
visible = True,
colors = colors,
labels = T_values_str,
)
critical_point = bqm.Scatter(
name = '',
x = [1.0],
y = [1.0],
scales = {'x': scale_x, 'y': scale_y},
default_opacities = [1.0],
visible = True,
colors = ['#2807a3'],
)
fig_115_001.marks = [
lines,
critical_point
]
right_block.children = [fig_115_001]
change_view_button = widgets.ToggleButton(
value=False,
description='Presentation mode (OFF)',
disabled=False,
button_style='',
tooltip='',
icon='desktop',
layout=widgets.Layout(
width='initial',
align_self='center'
)
)
change_view_button.observe(change_view, 'value')
prepare_export_fig_0_button = widgets.Button(
description='Export '+parameters[0][2],
disabled=False,
button_style='',
tooltip='',
)
prepare_export_fig_0_button.on_click(prepare_export)
prepare_export_fig_1_button = widgets.Button(
description='Export '+parameters[1][2],
disabled=False,
button_style='',
tooltip='',
)
prepare_export_fig_1_button.on_click(prepare_export)
prepare_export_fig_2_button = widgets.Button(
description='Export '+parameters[2][2],
disabled=False,
button_style='',
tooltip='',
)
prepare_export_fig_2_button.on_click(prepare_export)
prepare_export_fig_3_button = widgets.Button(
description='Export '+parameters[3][2],
disabled=False,
button_style='',
tooltip='',
)
prepare_export_fig_3_button.on_click(prepare_export)
prepare_export_fig_115_001_button = widgets.Button(
description='Export',
disabled=False,
button_style='',
tooltip='',
layout=widgets.Layout(
align_self = 'center',
)
)
prepare_export_fig_115_001_button.on_click(prepare_export)
tenperatures_text = widgets.HTML(
value="<div style='width:30px;text-align:left;display:inline-block;margin-left:30px;" \
+ "border: 5px solid #0079c4;opacity: 0.5'> </div>" \
+ " T = 0.8 Tc" \
+ "<div style='width:30px;text-align:left;display:inline-block;margin-left:30px;" \
+ "border: 5px solid #f09205;opacity: 0.5'> </div>" \
+ " T = 0.95 Tc" \
+ "<div style='width:30px;text-align:left;display:inline-block;margin-left:30px;" \
+ "border: 5px solid #21c400;opacity: 0.5'> </div>" \
+ " T = Tc" \
+ "<div style='width:30px;text-align:left;display:inline-block;margin-left:30px;" \
+ "border: 5px solid #850082;opacity: 0.5'> </div>" \
+ " T = 1.2 Tc" \
)
left_block.children = left_block.children + (
widgets.HBox([
prepare_export_fig_0_button,
prepare_export_fig_1_button,
prepare_export_fig_2_button,
prepare_export_fig_3_button
],
layout=widgets.Layout(
align_self = 'center',
)
),
)
right_block.children = right_block.children + (
prepare_export_fig_115_001_button,
tenperatures_text
)
main_block_115_000.children = [
change_view_button,
widgets.HTMLMath(
'$a: L^2 bar / mol \quad b: L/mol$',
layout=widgets.Layout(
align_self = 'center',
)
),
widgets.HBox([
left_block,
right_block,
])
]
figures.append(fig_115_001)
main_block_115_000